
views

Do the static test. Vigorously rub your amber on some wool for about 20 seconds to create static. Take a strand of hair and place the static-charged amber close to it. Real genuine amber should quickly attract the hair towards it, with the hair gently sticking to the gemstone. If no static is produced after rubbing on wool (ie it doesn't attract the hair) then you might have a piece of fake amber.
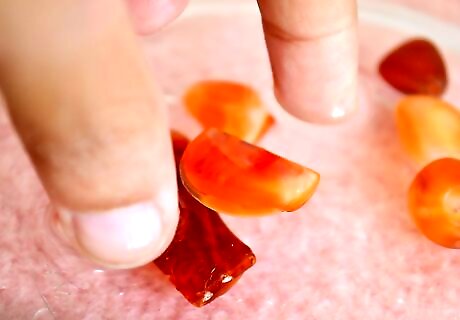
Know what to expect from each type of amber. Genuine amber is lightweight and warm to touch, not cold nor heavy like glass. Loose amber beads can be tested in salt water. Add 25g of salt to 200ml water in a glass and drop your amber into it. Genuine amber should float, not quickly sink to the bottom. Genuine amber reacts to ultraviolet light. Take your amber into a darkened room and shine a cheap UV torch on it. If it gently glows, it's real.

Check your amber piece for seams and mold marks, which indicate it may actually be made from plastic.

When you are about to buy a rough amber or carved amber, it can be easily tested with UV flashlight. When the uv flashlight apply, the color of the amber changes. If there is no changes in color of amber, it can be assured that it was synthetic (fake).

Another cracking the test of Amber: The reflections on the amber stone always show up downside. When you look the surface of amber, the images behind that stone reflect in downside position. You can easily test this way if you have excellent vision power.


















Comments
0 comment