'We Need to Wait, Trials Underway': Top Virologist on Availability of Covid Vaccination for Children
views
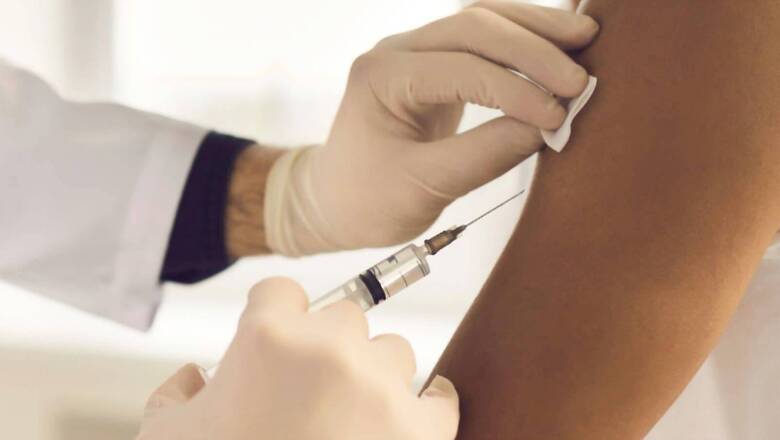
Amid rising concerns over the availability of Covid-19 vaccination for children, a top virologist Dr Gagandeep Kang has said inoculation of kids against coronavirus is not urgently required as the vaccine trials are still underway and the data for immunogenicity is awaited.
Only interim data has been given for approval to the Drugs Controller General of India (DCGI) for all vaccines against coronavirus. Hence, vaccination in kids can wait, said Kang in an exclusive conversation with India Today.
ALSO READ: As India Awaits Covaxin for Children, Here’s a Status Check of Vaccines for Those Below 18 Across Nations
An expert panel of India’s central drug authority has recommended granting emergency use authorization (EUA) to Bharat Biotech’s Covaxin for children and adolescents in the 2-18 years age group with certain conditions. If approved by the DCGI, it will be the second vaccine after ZyCoV-D to get EUA for use among those below 18 years. The National Technical Advisory Group on Immunisation (NTAGI) is looking at how ZyCov-D should be positioned for most optimum use.
The two-dose Covaxin will be administered to children with a gap of 28 days between the first and second dose. The vaccine dosage for children would be 0.5ml, the same as for adults. The SEC panel has recommended the company continue the study according to the approved protocols. The panel has also asked the company to submit the safety data, which also includes the data on adverse events, or side-effects every fortnight for the first two months and monthly thereafter.
ALSO READ: Covid Vaccine for Kids May Roll Out in November; Those with Weak Immunity to Get Priority
Kang said it is yet to be known how kids will react to the vaccines as its trial did not include children with co-morbidities. “We need to wait and try to develop a vaccine for kids that can offer protection against new variants instead of vaccinating them now,” she was quoted as saying by India Today and pointed out that children have already been exposed to the Covid-19’s Delta variant.
Kang further said vaccines developed using mRNA technology can be an option for children with co-morbidities as such vaccines produce the most effective antibody. She added that vaccinating kids with Bharat Biotech’s “inactivated vaccine” Covaxin would also be safe for them.
Speaking to India Today on the overall Covid-19 situation in the country, Kang said that the coronavirus pandemic has become “endemic” in India, and even if the country witnesses third wave as predicted, then it would be in the form of “small local outbreaks where the population is not yet exposed”.
On Sunday, Covid Task Force chief V K Paul said the government will take a final decision on vaccinating children and adolescents against coronavirus based on overall scientific rationale as well as the supply situation of vaccines available for those below 18-years-old.
Paul, who has been playing a key role in the government’s efforts in the fight against the coronavirus pandemic, also cautioned that even though infections are coming down and the second wave is subsiding, it will not be fair now to say that the worst is over since many countries have seen more than two waves.
Currently, three vaccines – Covishield, Covaxin, and Sputnik V – being administered in the country are only for those above 18 years of age.
Read all the Latest News , Breaking News and IPL 2022 Live Updates here.



















Comments
0 comment