views
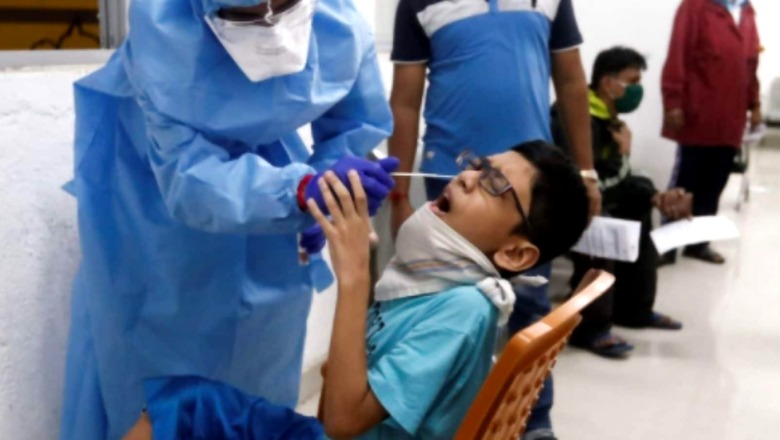
Indian drug regulator DCGI on Friday approved Zydus Cadila’s three-dose COVID-19 DNA vaccine for emergency use in adults and children aged 12 years and above, bringing in the sixth vaccine authorized for use in the country.
India has now approved its first vaccine for children, a timely move amid warnings of an upcoming third wave in the country, which some experts have warned could be deadlier towards children. While the Union Health Ministry has refuted that the next wave of the pandemic would prove more serious to the ‘vulnerable and unvaccinated’ population comprising children, it has, at the same time, augmented paediatric services across the nation as a preventive measure.
ALSO READ: ZyCoV-D: Decoding the Science behind India’s Plasmid DNA Vaccine & What Makes it Special
The company said it plans to manufacture 100 million to 120 million doses of ZyCoV-D annually and has started to stockpile the vaccine.
After news of the approval, Prime Minister Narendra Modi and Union Health Minister Mansukh Mandaviya lauded India’s fight against Covid-19, with another weapon in the country’s arsenal against the pandemic.
India is fighting COVID-19 with full vigour. The approval for world’s first DNA based ‘ZyCov-D’ vaccine of @ZydusUniverse is a testimony to the innovative zeal of India’s scientists. A momentous feat indeed. https://t.co/kD3t7c3Waz— Narendra Modi (@narendramodi) August 20, 2021
Double good news for the nation!@CDSCO_INDIA_INF approves the 1st DNA-based, needle-free #COVID19 vaccine in the world – ‘ZyCov-D’ of @ZydusUniverseMaking children of India COVID-safe, this vaccine can be used for individuals aged 12 and above. (1/2) https://t.co/YP0ZZylOS7
— Mansukh Mandaviya (@mansukhmandviya) August 20, 2021
'ZyCov-D' is the 6th approved #COVID19 vaccine in India, and the 2nd indigenously developed one.PM @NarendraModi ji's vision of #AatmanirbharBharat and Make in India delivers another significant accomplishment! (2/2)
— Mansukh Mandaviya (@mansukhmandviya) August 20, 2021
ALSO READ: ZyCoV-D, World’s 1st DNA Plasmid Covid-19 Vaccine Gets Govt Nod: All About India’s 2nd Homegrown Jab
The generic drugmaker, listed as Cadila Healthcare Ltd, applied for the authorization of ZyCoV-D on July 1, based on an efficacy rate of 66.6% in a late-stage trial of over 28,000 volunteers nationwide.
“Interim results from Phase-III Clinical Trials, in over 28,000 volunteers, showed primary efficacy of 66.6 per cent for symptomatic RT-PCR positive cases,” said a government release, adding that this has been the largest vaccine trial so far in India for COVID-19.
“This vaccine had already exhibited robust immunogenicity and tolerability and safety profile in the adaptive Phase I/II clinical trials carried out earlier. Both the Phase I/II and Phase III clinical trials have been monitored by an independent Data Safety Monitoring Board (DSMB),” it said.
ZyCoV-D is the world’s first plasmid DNA vaccine against the coronavirus. It uses a section of genetic material from the virus that gives instructions as either DNA or RNA to make the specific protein that the immune system recognises and responds to.
ALSO READ: Covid Cases in Bengaluru Kids: Karnataka Rushes To Improve Immunity, Sets up Centres
Zydus Cadila’s vaccine, developed in partnership with the Department of Biotechnology, is the second home-grown shot to get emergency authorization in India after Bharat Biotech’s Covaxin.ṇ
The drugmaker said in July its COVID-19 vaccine was effective against the new coronavirus mutants, especially the Delta variant, and that the shot is administered using a needle-free applicator as opposed to traditional syringes.
Earlier today, the Indian drug regulator’s subject expert committee had recommended emergency use approval for the vaccine. The committee had added that Zydus needs to submit additional data for the 2-dose regimen of its vaccine.
With inputs from Reuters.
Read all the Latest News, Breaking News and Assembly Elections Live Updates here.


















Comments
0 comment