
views
X
Research source
If you ever need to test for the presence of hydrogen gas, this is an easy (and fun) method to use.
Creating Hydrogen Gas

Gather the necessary materials. To create a hydrogen gas you will need hydrochloric acid, magnesium strips, a test tube, a match, lab gloves, and eye protection. When you place a metal into hydrochloric acid it will react to form hydrogen gas and a metal chloride compound. You only need about 20 mL of dilute hydrochloric acid (about 2 molar). Magnesium strips can be purchased online. Safety is important so be sure to wear gloves and eye protection throughout the whole experiment. Adult supervision is required for this experiment.
Create the right environment. Ideally, this experiment will be performed within a chemistry lab. Safety equipment such as a fire extinguisher, eye wash station, and safety shower should be easily accessible. The room should also be open and well-ventilated. Do not perform this experiment by yourself in your bedroom. Be sure to have an adult around to supervise.

Add hydrochloric acid to test tube. Once you have gloves and eye protection on, pour about 20 milliliters (0.7 fl oz) of hydrochloric acid into the test tube. You don’t need exactly 20 milliliters (0.68 fl oz), but pour just enough hydrochloric acid until there is about 2 centimetres (0.79 in) in the bottom of the tube. If you are diluting the acid always add the acid directly to the water. If you add the other way around, the acid can explode and cause injury. Note that hydrochloric acid is corrosive and releases an acidic mist.

Add a magnesium strip to the test tube. Drop the magnesium strip into the tube with the hydrochloric acid. The solution should start to bubble as soon as the metal comes into contact with the liquid. These bubbles are the hydrogen gas. You can add more than 1 strip to accelerate the formation of hydrogen.
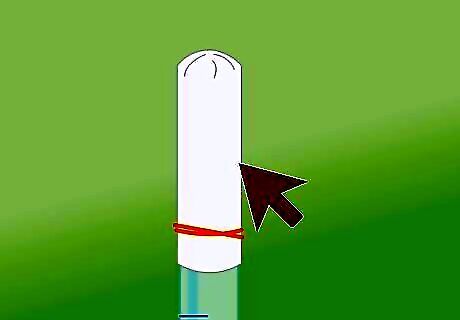
Cover the tube securely with plastic. Place some plastic wrap over the top of the test tube immediately after adding the metal. Rubber band the plastic in place to cover the tube as tightly as possible so the hydrogen gas doesn’t slip out into the air. Because the gas is less dense than the liquid it will rise to the top of the test tube as it is created. You can also cover the tube with another test tube or simply put your thumb on top. Let the reaction go for at least a minute to build up the amount of gas in the tube.
Testing for the Squeaky Pop

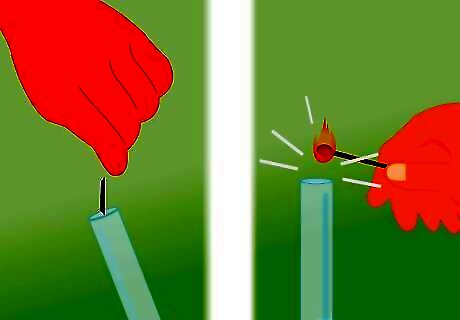
Light a match. You don’t have to use a match for this, but you need something that is lit on fire. You can use a lighter if you would prefer. Whatever flame source you are using, light it and have it ready to put near the tube as soon as you remove the covering. Adult supervision is essential while working with an open flame.
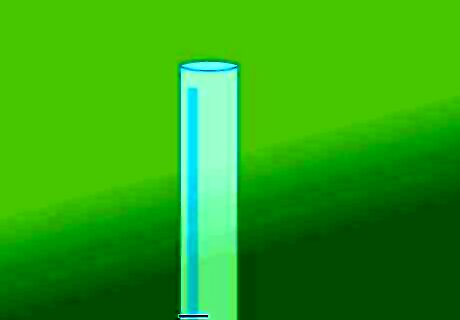
Remove the covering from the tube. Remove the rubber band securing the plastic in place and then lift the plastic off the top of the tube. Proceed immediately to the next step after uncovering the tube so you don’t let too much of the gas escape. As soon as the covering is removed, the hydrogen will start to escape into the surrounding air.
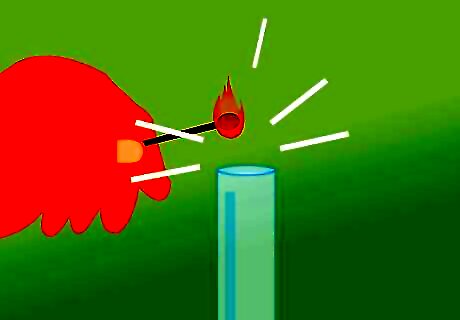
Place the match near the top of the tube. Bring the lit match to the tube immediately after uncovering it. Once the flame comes in contact with the gas, it will likely be blown out, but you will hear the characteristic “squeaky pop” at the same time. Use caution during this process. You are working with flammable gases and open flames. Have adult supervision when you do this. If you don’t hear the pop, there likely isn’t enough gas in the tube. Recover it and let the reaction go for a longer amount of time. Make sure the solution is bubbling.
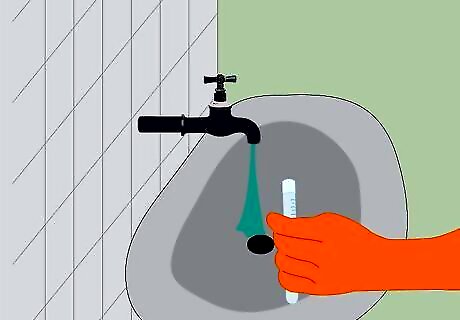
Dispose of everything properly. Remove the metal from the test tube using tweezers. Dilute the hydrochloric acid in about 4 litres (1.1 US gal) of water. Then, pour the diluted solution down the drain while running some water at the same time. Rinse the metal in the sink and then dispose of it in the trash. You can also save the metal and use it again to repeat the experiment if you would like. Be sure to find out the regulations in your region for disposing of hydrochloric acid, as they vary.
Utilizing the Experiment
Create learning goals. The primary objective for teaching this type of experiment is to talk about chemical reactions. This particular experiment is an example of metals reacting with acids to create gases. Students will be able to see with their own eyes how reactive the metal is and how quickly gas is produced by the reaction. A secondary goal could be to discuss lab safety and working with flammable gases.
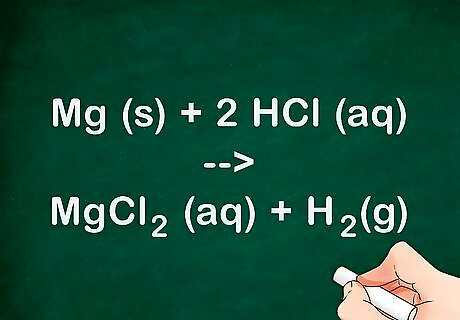
Discuss the reaction that occurred. Talk about how the acid reacts with the metal. When discussing chemical reactions you want to talk about the reactants and how they combine together to form the various products. Talk about the states the products and reactants exist in. The magnesium is a solid that reacts with the aqueous hydrochloric acid to form hydrogen gas and aqueous magnesium chloride. The chemical equation for this experiment is: Mg (s) + 2 HCl (aq) --> MgCl2 (aq) + H2(g). This reaction is also known as a single replacement reaction, meaning that the magnesium replaces the hydrogen bound to chlorine to form magnesium chloride.

Talk about the applications of the experiment. This type of experiment is a good way to test for the presence of small amounts of hydrogen gas. Remember, hydrogen is flammable so if you suspected that there was a lot of hydrogen gas present, you wouldn’t want to do this test. In a controlled laboratory setting where you know the experiment is supposed to produce hydrogen, you can use this test to confirm.
Expand the experiment with other metals. Provide the students with other types of metals such as iron, aluminum, copper, and zinc. Do the exact same procedure and then test for the presence of hydrogen gas. You’ll notice that not every type reacts with hydrochloric acid to form a hydrogen gas. Do the same experiment but warm the acid a bit. You’ll notice that more of the metals will react to form hydrogen gas when the acid is warm. Discuss the results with your students.

















Comments
0 comment