views
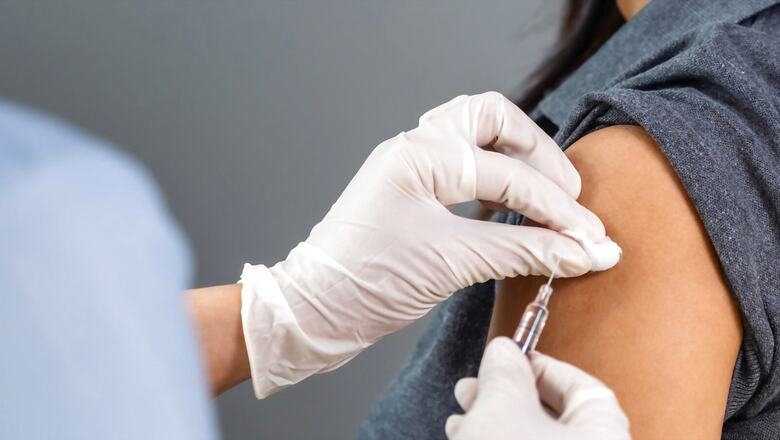
Reliance Life Sciences has applied for regulatory approval to begin early-stage human trials of its COVID-19 vaccine candidate on Thursday, according to reports.
The application for phase I trial of Reliance Life Sciences’ recombinant protein-based vaccine candidate will be reviewed by the Subject Expert Committee (SEC) on August 26, according to an Economic Times report.
The aim of the clinal trials is to obtain reliable information on safety, tolerability, pharmacokinetics (PK), and mechanism of action of drugs with the objective of determining the maximum tolerated dose (MTD), sources were quoted as saying.
Speaking on the duration of the trial, sources said, “Phase I trials are usually conducted for 58 days to check the tolerated dose strength. Once it’s done, the company can approach for PhaseII/III trials.”
Read all the Latest News, Breaking News and Assembly Elections Live Updates here.


















Comments
0 comment