views
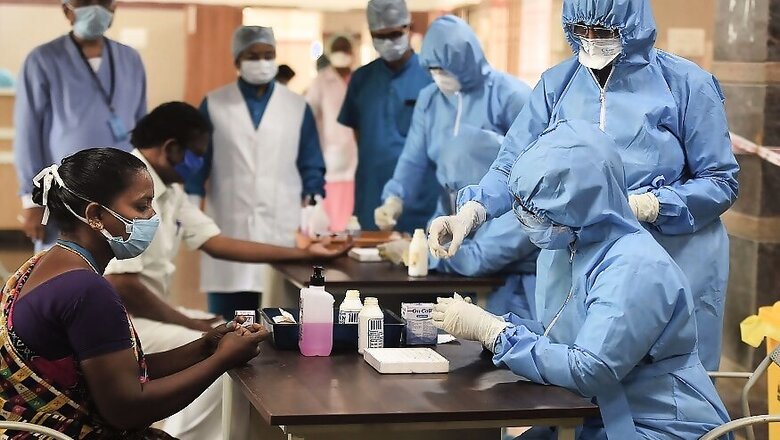
Chennai: Countering complaints of defective rapid testing kits, China's Wondfo Biotech has said that variations in results could be due to the timing of the tests as it is an important factor in determining accuracy. It also pointed out that the firm's products have the approval of the National Institute of Virology (NIV) in Pune.
In an exclusive statement to CNN-News18, the Chinese firm said that it was surprised and disappointed with the government decision. The Centre had initiated the rapid test kits as a surveillance tool to monitor the spread of coronavirus, but on Monday, advised against its use after it found results showing "wide variation in their sensitivity, despite early promise of good performance for surveillance."
"...the Wondfo SARS CoV-2 Antibody Test (Lateral Flow Method) is one of the first tests to receive an import licence from India, and has been validated and approved by the ICMR through National Institute of Virology-Pune. As a company, quality is our highest priority and we are confident in the quality of our products which meet standards in China and countries that we are exporting to..." read the statement.
The Indian Council of Medical research had sent out advisories to state governments on Monday to stop the use of kits from Wondfo and another supplier Zhuhai Livzon Diagnostics, just a little over 10 days after it clarified that the rapid test kits were no confirmatory tools but can be used as a surveillance tool in the battle against Covid-19.
"States have now been told to not use these kits and return them to be sent back to the suppliers," said ICMR.
In its statement, Wondfo Biotech has asserted that the timing of the tests is an important factor determining accuracy. "The use of appropriate and pertinent testing methods varies over the course of viral infection. Based on the timing of the appearances of the Ribonucleic Acid (RNA) indicating live virus, antigens and the two types (IgG/IgM)of antibodies in the human body, different testing methods are used to ensure the accuracy of test results.”
It is in this context that the response of one of suppliers Wondfo, asserting the validity of its products and advancing its licenses in China, needs to be seen. In its statement, Wondfo also assured the world community that it would "continue to be transparent about its measures.."
Meanwhile, the pricing of these kits has also turned into a contentious debate. The Indian Government has said that it has not lost any money in the purchase of rapid test kits. In an importer-distributor dispute ruled on April 24, the Delhi High Court said the rapid test kits should not be sold beyond Rs 400 inclusive of GST. It is also interesting to note that the exporter/supplier to ICMR will receive only Rs 11.25 crore of the Rs 30 crore to be paid by ICMR for 5 lakh test kits.
Political and national affairs commentator Sumanth Raman, who is also a medical expert, while responding to Wondfo’s statement, told CNN News18: “It is inconceivable that the rapid test kits would not have been tested in India for its results at different levels/ times, and circumstances.” He added: “But, it is possible that one particular batch could have been faulty. Secondly, it is said that the kits were tested by NIV-Pune. So, NIV-Pune has to respond now...”
Tamil Nadu has been one of the states, among many, to procure the kits. In the Delhi High Court order, it is mentioned that Tamil Nadu awaits supply of 26,000 kits. On Monday, the southern state had announced that it was adhering to the directions of ICMR and sending back the rapid test kits.



















Comments
0 comment