views
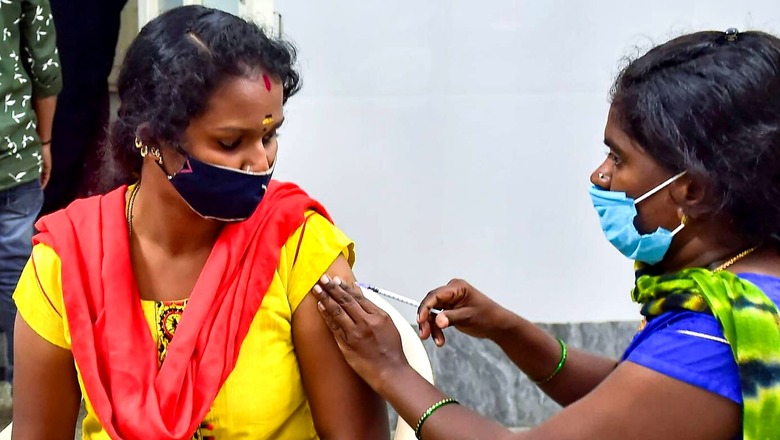
Is my immunity against COVID-19 waning? Should I be worried about COVID reinfection? These are the two primary concerns that have made ‘booster dose’ a part of drawing room conversations.
While there is no clarity on how the government plans to kick-start the drive to provide additional doses of COVID-19 vaccines, global studies are now showing that these could be the need of the hour—if not for everyone, then at least for healthcare workers, elderly and vulnerable population.
Globally, signals of waning protection first started showing in people above 65 years of age—the age group that was vaccinated on priority as soon as vaccines were launched by the end of 2020 or early 2021.
But before we proceed further, let’s understand how COVID-19 vaccines provide us immunity.
Vaccines, usually, provide immunity at two levels—first, by stopping the pathogen from causing infection (the COVID vaccines presently in use cannot do that) and second, if the pathogen has caused infection, then controlling its progression at the initial stages (which is what the vaccines are doing).
Vaccines train our body’s neutralising antibodies to recognise and kill the virus as soon as it enters the body. But if they fail to kill, vaccines train our memory B and memory T cells—which are in charge of the body’s defence system—to fight against the virus and flush it out.
Experts believe that somehow the training of one of these three systems (neutralising antibodies, memory B and memory T cells) must be getting affected.
Immunity Offered by Covishield Waning?
Researchers at Public Health England posted a preprint in September which builds a strong case for older adults and those with co-morbidities to be prioritised for booster doses if administered Covishield, the viral-vector vaccine from AstraZeneca, which is called ‘Vaxzevria’ in the UK.
The dip in vaccine effectiveness was seen in over 20 weeks from inoculation. A higher dip was observed in the 65+ age group and in the 40-64 age group with underlying medical conditions, compared to healthy adults.
“Vaccine effectiveness against symptomatic disease peaked in the early weeks after the second dose and then fell to 47.3 and 69.7 by 20+ weeks against the Delta variant for Vaxzevria and Comirnaty, respectively,” the study said.
The good news, as per the study, is that there is limited waning of vaccine effectiveness against hospitalisation and death even after 20 weeks post-vaccination.
However, the key take away from the study is that immunity starts waning roughly after five months (or 20 weeks) of taking the second dose.
India started jabbing healthcare workers against COVID-19 in January this year. Going by the study, the immunity should start waning after September, considering the 84-day gap between the first and second dose of Covishield. Bharat Biotech has already said that the ideal time for a booster dose of Covaxin is six months after the second dose.
The booster policy in the United Kingdom, which has used AstraZeneca’s Vaxzevria in their immunisation plan, states that “the booster is being offered at least 6 months after your last dose.”
“The protection against severe disease from the first 2 doses seems to decline very slowly. So don’t worry if your booster vaccine is given a short time after the 6 months time-point. The booster dose should help to extend your protection into the next year.” However, with the arrival of a new variant Omicron, the UK has reduced the waiting period for the booster to three months after the second dose for those over 40 years of age and in high-risk group.
America’s CDC offers boosters six months after the second dose of Pfizer or Moderna vaccines and two months after the J&J vaccine. The country did not use AstraZeneca’s vaccine, which has been India’s prime vaccine.
What’s the Status in India?
Several other studies conducted globally are showing that the rate of infections, hospitalisation and deaths are rising among the vaccinated.
While majority of these studies have been done on mRNA vaccines, there is an urgent need to collate and analyse the data on vaccines used in India.
The National Technical Advisory Group on Immunisation (NTAGI) met on December 6 to deliberate on India’s booster programme but no decision was taken.
The UK’s health regulator has approved the use of AstraZeneca vaccine as a COVID booster following which the Serum Institute of India has now applied to Drug Controller General of India for approval of Covishield as a booster dose.
Over the last few years, the Indian drug regulator has been referring to case studies and data used by international regulators before approving medical products here; however, no decision has been taken on SII’s application yet.
A senior official in the Ministry of Health and Family Welfare believes that the focus is presently on increasing primary vaccine coverage before introducing boosters.
For the record, more than 85 per cent of the adult eligible population has taken the first dose of a COVID-19 vaccine and more than 50 per cent is fully vaccinated with two doses.
What Can be Done?
With the country sitting on an unutilised stock of over 20 crore vaccines—bulk of which is Covishield—the government should allow private hospitals to start administering doses to elderly and vulnerable groups. Healthcare workers, who were vaccinated before the elderly, should again be prioritised and given booster shots.
Moreover, several experts suggest that if the government doesn’t want to offer booster doses to everyone, it should decide a cut-off for the antibody levels.
Those who do not fall under priority groups such as healthcare workers, elderly or vulnerable can get boosters if their antibody levels are below the set cut-off at private hospitals.
The government can announce a range of antibody levels deemed fit for fighting COVID and preventing hospitalisation and severe infection. Those willing to take boosters can get the antibody test done at certified labs. However, before making this move, more clarity is required on what are the appropriate levels for COVID antibodies.
The message from science is clear, especially in the wake of Omicron. India needs to focus on collection and analysis of data on COVID-19 vaccines.
Read all the Latest Opinions here

















Comments
0 comment