views
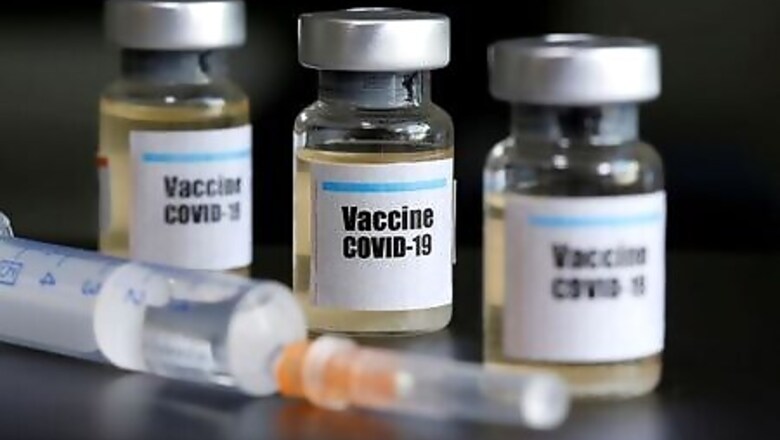
The U.S. Centers for Disease Control and Prevention (CDC) has asked state public health officials to prepare to distribute a potential coronavirus vaccine to high-risk groups as soon as late October, according to documents published by the agency.
The timing has taken on political importance as U.S. President Donald Trump seeks re-election on Nov. 3, after committing billions of federal dollars to develop vaccines against COVID-19, which has killed more than 185,000 people in the United States.
Pfizer Inc said on Thursday it should know by the end of October whether a COVID-19 vaccine it is developing with German partner BioNTech SE is safe and effective.
The U.S. drugmaker said it will seek approval immediately if trial results are positive. It has already manufactured hundreds of thousands of doses.
Top U.S. infectious diseases expert Anthony Fauci said on Thursday having a vaccine ready by the end of October is possible, but he was not counting on it.
“These are all guesstimates,” Fauci told CNN, when asked about Pfizer’s comments, adding that most experts project a vaccine will be ready by November or December. “It is conceivable that you can have it by October, though I don’t think that that’s likely.”
The CDC “provided states with certain planning assumptions as they work on state specific plans for vaccine distribution, including possibly having limited quantities of vaccines in October and November,” an agency spokeswoman told Reuters.
The New York Times had earlier reported that the CDC had contacted officials in all 50 states and five large cities with vaccine planning information.
The documents https://int.nyt.com/data/documenttools/covid-19-vax-planning-assumptions-8-27-2020-final/6fc8a9ec0c3e5817/full.pdf put online by the New York Times showed the CDC is preparing for one or two vaccines for COVID-19 to be available in limited quantities as soon as late October.
The vaccines would be made available free of cost first to high-risk groups including healthcare workers, national security personnel, and nursing home residents and staff, the agency said in the documents.
Regulators around the world have repeatedly insisted that development speed will not compromise vaccine safety, as quicker results would stem from conducting parallel trials that are usually done in sequence. Such reassurances have not convinced everyone that political pressure will not play a role.
Fauci told CNN he is confident the Food and Drug Administration and independent advisory panels of experts will all review data on vaccine candidates to make sure they are safe and effective.
Preliminary results of a survey conducted over the last three months in 19 countries showed that only about 70% of British and U.S. respondents would take a COVID-19 vaccine if available, Scott Ratzan, co-leader of a group called Business Partners to Convince, told Reuters in August.
AstraZeneca Plc, Pfizer and Moderna Inc are among those farthest along in the race to develop vaccines to prevent COVID-19 infection or limit severity of the illness. Their candidates are all in late-stage clinical trials.
Last month, U.S. health officials said the CDC was executing an existing contract option with McKesson Corp to support potential vaccine distribution.
CDC Director Robert Redfield has asked state governors to expedite McKesson’s requests for building vaccine distribution centers and to consider waiving requirements that would stop them from becoming fully operational by Nov. 1, according to a recent letter seen by Reuters.
Disclaimer: This post has been auto-published from an agency feed without any modifications to the text and has not been reviewed by an editor

















Comments
0 comment