views
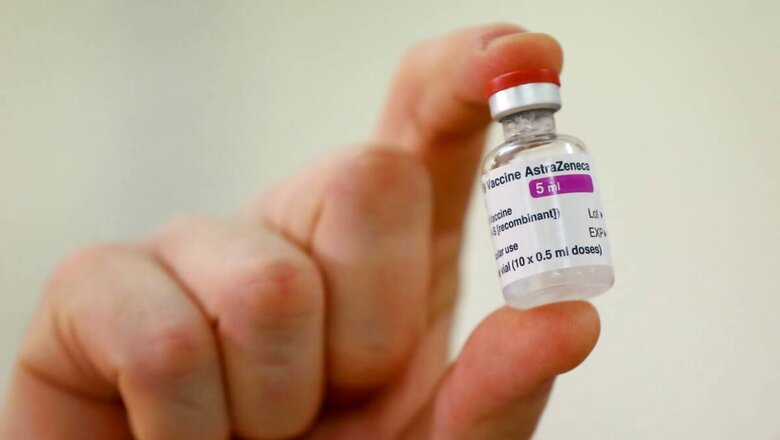
AIIMS director Randeep Guleria on Sunday said India will use only Oxford-AstraZeneca vaccines in the first phase of Covid-19 vaccination and Bharat Biotech’s Covaxin is only a back-up in case the more infectious UK virus variant gets out of control. Both vaccines have been granted emergency use approval by the Drugs Controller General of India (DCGI) but the nod to Covaxin has triggered concerns since the Hyderabad-based Bharat Biotech is yet to complete phase III human trials.
Speaking to News18, Guleria said any person who develops side-effects after being administered Covaxin will be eligible for compensation. “India will start procuring Covaxin if the UK virus variant situation explodes or after the trials are completed, whichever happens first. Any person receiving Covaxin will get compensation in case of side effects just as the way if happens in clinical trials,” he said.
Guleria’s statement came as Congress and Left leaders questioned the nod to Bharat Biotech when its Phase III trials are yet to be completed. Congress leader Shashi Tharoor said the approval was “premature”, while party colleague Anand Sharma asked the government to explain why mandatory protocols and verification of data has been dispensed with. CPM leader Sitaram Yechury said information on the trials and results should be make public to build confidence among people.
Listen in to what Dr Randeep Guleria, AIIMS Director had to say over by when people could get the initial shots of #Covid19 vaccine.Join the broadcast with @snehamordani. pic.twitter.com/eaV1RGAvmt
? News18 (@CNNnews18) January 3, 2021
“The announcement about the two vaccine candidates should be accompanied by full disclosure of the minutes of the meetings, information about all the trials and results in order to build confidence in the people. This has been done globally. Our government should do so too,” Yechury said on Twitter. “Any attempt to short-circuit the regulatory process for political gains will damage the good reputation built by Indian pharma over the years.”
Guleria said politics over the approval is unfortunate. “Scientists don’t know politics. They have done their work relentlessly,” he said. He added that the chances of the vaccine working against the mutated virus variant is “very high”.
“We are worried considering what happened in US and Europe. We don’t want to land up in such a situation. We should have faith in our scientists, they worked very hard,” he said, referring to cases of allergic reactions among recipients in the West.
The Phase III human clinical trials of Covaxin began mid-November, targeted to be done in 26,000 volunteers and it is the country’s first and only Phase III efficacy study for a COVID-19 vaccine, and the largest phase III efficacy trial ever conducted for any vaccine in India, a press release from the vaccine maker had said on Saturday night.
Covaxin has been evaluated in approximately 1,000 subjects in Phase I and Phase II clinical trials, with promising safety and immunogenicity results, with acceptance in international peer reviewed scientific journals. It is being developed by Bharat Biotech in collaboration with the Indian Council of Medical Research (ICMR) – National Institute of Virology (NIV).
Read all the Latest News, Breaking News and Coronavirus News here
















Comments
0 comment