views
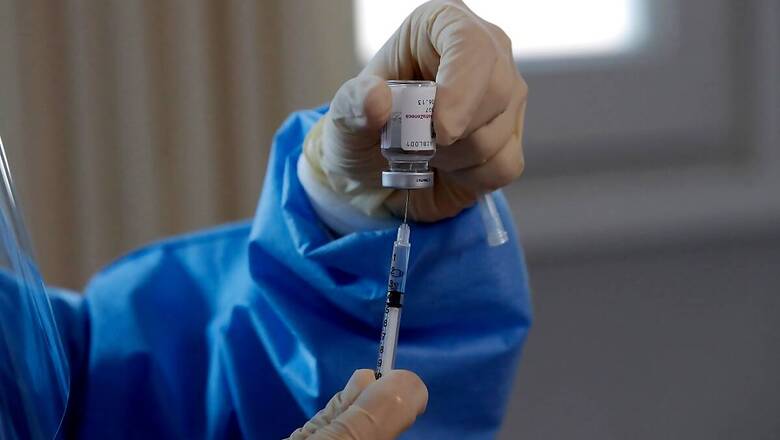
In part-I of Vaccines Demystified, we had a close look at Pfizer-BioNTech, Moderna, Johnson and Johnson, and Oxford-AstraZeneca vaccines against Covid-19. In a U-turn, on 2 March, France approved the AstraZeneca vaccine for over-65 year olds. It is being expected that Germany could do announce a similar decision soon. However, according to some reports, a section of French doctors have expressed displeasure over the recent move.
In yet another signal of intent of significant departure from previously established policy, the European Medicines Agency (EMA) of the European Commission is considering if it should start granting “Emergency Use Authorisation (EUA)” to Covid-19 vaccines for member states of the European Union (EU). Till now, the EMA has been granting only “Conditional Market Authorisation (CMA)” to the eligible Covid-19 vaccines.
Difference between CMA and EUA
As per the European Council, Conditional Market Authorisation or CMA follows a controlled and robust framework providing safeguards that emergency use authorisations might not. The CMA ensures that rigorous monitoring is put in place including legally binding post-approval obligations of the vaccine manufacturer, including pharmacovigilance, manufacturing controls and batch controls etc. Naturally, it is a much lengthier and time-consuming process compared to EUA. However, it is more rounded and ensures plans are put in place for present and future to ensure a higher level of protection of citizens. CMA is valid for one year after which it needs to be renewed. With CMA, the liability rests with the manufacturer of the vaccine or holder of the CMA. It is a key element of vaccine strategy in the EU.
Emergency Use Authorisation or EUA, on the other hand, is not actually an authorisation of the vaccine but authorisation of a temporary use of a vaccine under specific conditions in an emergency situation, as long as the emergency circumstances apply, like in this pandemic. The administrative and civil liability is removed from the manufacturer or the holder of an EUA. The medicine or vaccine, however, remains unlicensed and cannot be put on the market, contrary to a (conditional) market authorisation.
The grant of EUA for EU member states had been traditionally restricted by Brussels to “most urgent” situations, like the anticancer drugs. Individual member states can, however, extend EUA to vaccines in their territory, but the responsibility then shifts to the member state approving EUA.In short, EUA is quicker, but absolves the manufacturer of responsibility should problems happen.
EU has granted Conditional Market Authorisation to Pfizer-BioNtech, Moderna and Oxford-AstraZeneca for use in the EU member states, and evaluation of Johnson and Johnson vaccine is happening as we speak. However, many other countries, including the UK, the US and India have taken the path of Emergency Use Authorization. A move to EUA can speed up vaccination approval in the EU. This week, the UK crossed 20 million vaccinations causing envy in French and German press, and much of this is being seen in the European media as a result of the EUA and Brexit.
Tracking Covishield
This is essentially the Oxford-AstraZeneca vaccine being manufactured by the Serum Institute of India. In India, it was launched after it received the Emergency Use Approval by the Drugs Controller General of India (DGCI) in January 2021 without formal phase-3 trial on Indian population. Since then, a number of countries have ordered and expressed interest in ordering Covishield under the Vaccine Maitri initiative or directly from the company.
On February 15, the World Health Organisation granted Emergency Use Listing (EUL) to Covishield for individuals over 18 years of age, including for those over 65 years of age. This EUL allows two full doses to be administered between four to 12-week interval.
This enables the company to export nearly 20 million doses to countries under the COVAX initiative of the WHO. The countries covered would include African and other low-income nations that have traditionally been feared to lag behind richer nations in global vaccination drive. Another manufacturer of the Oxford-AstraZeneca vaccine, Korean company SKBio, has also been approved by the WHO for global rollout under the COVAX initiative.
The Serum Institute of India has also teamed up with GAVI vaccine alliance and Bill and Melinda Gates Foundation to supply 200 million vaccine doses this year, at $3 per dose. The UK has ordered 10 million doses of AstraZeneca vaccine from Serum Institute of India, after a visit from the UK’s trade secretary Liz Truss. The EU drug regulator has also started auditing the manufacturing site of Serum Institute of India, which is a prerequisite step in the EU legislation before ordering vaccine supplies. It is being anticipated that Covishield could soon be supplied to EU member states.
Tracking Covaxin
Covaxin is an inactivated SARS-CoV2 virus antigen, developed indigenously by Bharat Biotech in conjunction with the Indian Council of Medical Research (ICMR) and the National Institute of Virology (NIV). It had received criticism from some scientists and medical professionals after it was given EUA by the DCGI in January without a proper phase-3 trial. It was highlighted that the phase-1 trial was done only on 375 persons, while 380 participants aged between 18-60 years were enrolled for the phase-2 trials.
The vaccine was given initially to Indian healthcare workers workers; later, its use was expanded to include other frontline workers and general public. The company on Wednesday announced that the phase-3 clinical trials, which involved 25,800 participants, have demonstrated an interim efficacy of 81 per cent. The vaccine is being injected intramuscularly in two doses, 28 days apart.
A number of countries have ordered or expressed interest in ordering Covaxin which has also found a place in the Vaccine Maitri programme. This week, Prime Minister Narendra Modi received his first dose of Covaxin at the All India Institute of Medical Sciences, Delhi.
Coming up next: Part-3 of the Vaccines Demystified series, which will explore Novavax, Sanofi-GSK, Sputnik V, Sinovac, Sinopharm and other vaccines.
Read all the Latest News, Breaking News and Coronavirus News here


















Comments
0 comment