views
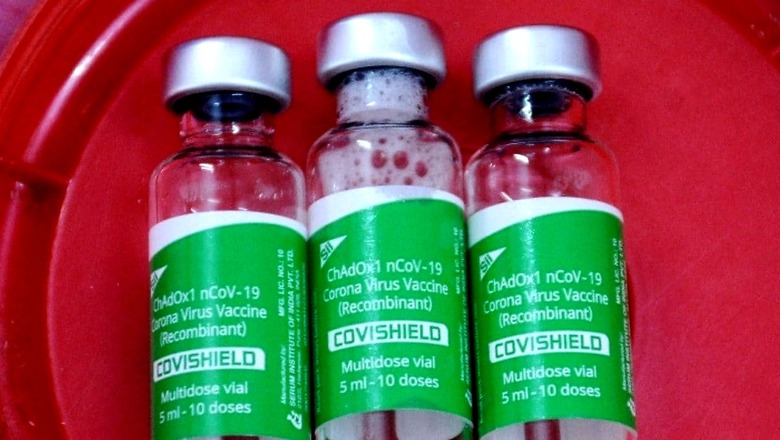
AstraZeneca’s vaccine has demonstrated the lowest incidence of side effects in India compared to the global average, a top government official involved in the national roll-out of Covid-19 vaccination told News18.
The official, who requested anonymity, said India had reported around 80,000 adverse events from Covishield out of a staggering 180 crore doses administered. However, he said, 98 per cent of these events included mild local reactions such as fever, swelling on site of injection or pain.
“But only around 50 cases witnessed Thrombosis with Thrombocytopenia Syndrome (TTS) — a rare side effect caused by the jab — of the 180 crore doses administered against 4 to 10 cases (out of every 10 lakh doses) globally,” the official said.
“Roughly, the chances of TTS stood at 0.000003 per cent with Covishield in India whereas globally, the risk stood at 0.0004 per cent,” the officer estimated.
Such estimates come amid the Anglo-Swedish drugmaker announcing that it has started the global withdrawal of its Covid-19 vaccine, days after the company admitted that its vaccine has the potential to cause a rare side effect called TTS.
TTS is a blood disorder characterised by blood clots forming in small blood vessels throughout the body. Thrombocytopenia refers to a low platelet count in the blood.
AstraZeneca’s vaccine, known as Vaxzevria globally, was manufactured by the Serum Institute of India (SII) in India and sold under the brand name Covishield.
To contain panic and alarm, the official clarified that “the vaccine does not exhibit any long-term effects on the human body”.
If such a side effect were to occur, it would most likely manifest during the initial dose, he said.
“However, if it has not occurred by then, it is improbable to arise later, ensuring your safety,” the official said, adding: “There is no reason to doubt the efficacy of the vaccine or anticipate any adverse events in the future.”
Explaining why the incidence of side effects was low in India, the official added that “the genetic makeup of people from South Asia (South Asians) makes them less likely to develop thrombotic and thrombocytopenia syndrome (TTS) compared to other populations”.
On being asked if the lower rate of side-effects could be because of under-reporting, the officer added: “In general, the advantages of vaccination far outweigh the minimal risk of harm. Nevertheless, as a precautionary measure, India has been consistently monitoring the emerging signs of harm.”
In response to widespread apprehension regarding TTS, the Serum Institute of India, maker of Covishield, said on Wednesday that the packaging of its product had “fully disclosed all uncommon to exceedingly rare side effects,” including TTS.
“We fully understand the ongoing concerns and it is crucial to emphasise our commitment to transparency and safety. From the outset, we have disclosed all rare to very rare side-effects, including Thrombosis with Thrombocytopenia Syndrome, in the packaging insert in 2021,” a spokesperson of SII said.
It said that the company stopped the manufacturing of additional doses of Covishield in December 2021 in India.
Stay updated with live coverage of Lok Sabha Election 2024 Phase 3 Voting In Karnataka And Gujarat on our website. Get the latest updates, polling trends, result dates and more.




















Comments
0 comment