views
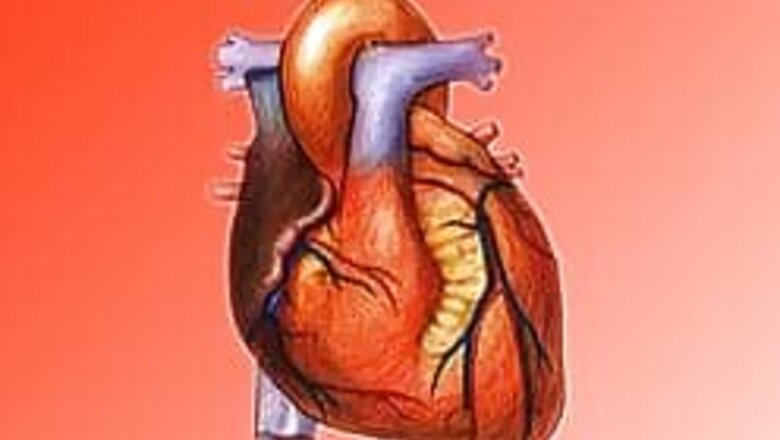
Washington: A Massachusetts company received federal approval on Tuesday to sell up to 4,000 artificial hearts a year, though the number of devices implanted annually will likely be far smaller.
The hearts would be used only in patients who are close to death and have no other treatment options.
The Food and Drug Administration (FDA) granted Abiomed Inc, a humanitarian exemption allowing it to sell the devices, agency spokeswoman Susan Bro said.
The actual number of the devices, called the AbioCor, to be implanted likely will be small — between just 25 and 50 a year, Bro said.
So far, the artificial heart has been tested in only 14 men.
Two died from the operation, and another never regained consciousness.
The rest survived only an average of five months, with one exception: a man who lived 17 months, until the mechanical heart wore out.
The company said earlier that it would begin implanting the artificial hearts at five hospitals around the country, once doctor training is complete.
The devices are fully contained within the chest, with no outside wires.
Abiomed is targeting men but not precluding women with heart failure who are too sick for a heart transplant, have exhausted other options and are likely to die within a month.
The current device is too large for about 90 per cent of US women and many men so the company is developing a smaller version.
In 2005, an FDA panel of outside experts voted against recommending Abiomed be given permission to sell the device in limited numbers.
At the time, the experts expressed concern that many AbioCor recipients suffered severe strokes, some fatal that compromised their final weeks.
The company has since redesigned the design of the cuff of the device to prevent two bars from coming into contact with human tissue.
That contact was believed to be the cause of the strokes in the first test patients, said the company's president and chief executive officer, Michael R Minoque.
The company also hopes to implant the hearts in patients who can be treated with blood-thinning drugs, further reducing the risk of stroke, Minogue said in a recent interview.
"We want to focus on getting the right patients and getting them home, so whatever that number is, that is what it will be," Minogue said.
The implant is expected to cost about $250,000. It is unclear whether insurance would cover it.
Abiomed eventually hopes 10 medical centers would be equipped to implant the hearts.
The devices themselves now last about 18 months, or longer than the patients receiving them can be expected to live.
On Tuesday, Minogue said that the first surgeries to implant the artificial hearts would take place in six to eight months.
"It's allowed us to move forward, to make science fiction 'science fact,'" Minogue said of the FDA action adding, "We have a product called AbioCor that allows us to remove a person's heart and keep them alive."
The electrical current to power the device passes through the skin, without the need for wires, from a battery belt worn by the patient. The belt also can be plugged in.
The device's internal batteries can go more than an hour without recharging, allowing patients to shower.

















Comments
0 comment